Tag: CCI
 Hackers Targeting Covid-19 Vaccine Cold Chain, Warns IBM
Hackers Targeting Covid-19 Vaccine Cold Chain, Warns IBM
Tech giant IBM on Thursday said its team has uncovered a global phishing campaign targeting organisations associated with a Covid-19 cold chain. ....
 BioNTech CEO Recounts Origin Story Of Covid Vaccine
BioNTech CEO Recounts Origin Story Of Covid Vaccine
Scientists from BioNTech and Astra Zeneca which are at the bleeding edge of coronavirus vaccine development revealed little known details of the origin stories of their frantic race that began early this year, at a United Nations Covid-19 summit on Friday. ....
 British Indian Couple Among 1st In World To Receive Covid Vaccine
British Indian Couple Among 1st In World To Receive Covid Vaccine
As UK began one of the largest vaccination drives, an Indian-origin couple in their 80s became one of the first few people to receive the recently approved Coronavirus vaccine. ....
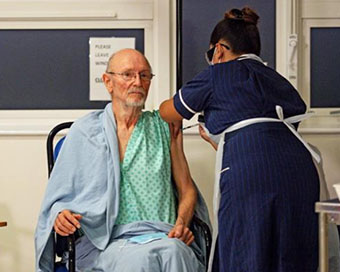 William Shakespeare 2nd Person In UK To Receive Pfizer-BioNTech Covid Vaccine
William Shakespeare 2nd Person In UK To Receive Pfizer-BioNTech Covid Vaccine
The UK has begun one of its largest vaccination drives and 81-year-old namesake of the Bard of Avon, William Shakespeare, became the second citizen in the West to receive the Pfizer-BioNTech Coronavirus vaccine. ....
 BCCI Boss Ganguly & Co. To Continue As SC Defers Hearing To 2021
BCCI Boss Ganguly & Co. To Continue As SC Defers Hearing To 2021
Indian cricket board president Sourav Ganguly, secretary Jay Shah, and joint secretary Jayesh George, whose tenures have ended, would continue at least till the third week of January, when the Supreme Court would next hear the cricket reforms case. Besides fixing the next listing, the court on Wednesday disposed of a "substantial number of" the 102 applications attached with the case.....
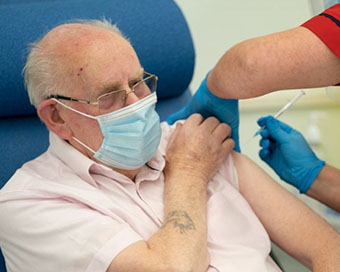 UK Warns People With Serious Allergies To Avoid Pfizer Covid Vaccine After Two Adverse Reactions
UK Warns People With Serious Allergies To Avoid Pfizer Covid Vaccine After Two Adverse Reactions
The UK's medical regulator has advised that anyone who has a history of significant allergic reactions to medicines, food or vaccines should not receive the Pfizer/BioNTech vaccine currently in use in the country, according to media reports. ....
 UN Chief Antonio Guterres Says He'll Take Covid Vaccine
UN Chief Antonio Guterres Says He'll Take Covid Vaccine
UN Secretary-General Antonio Guterres has confirmed that he will take a Covid-19 vaccine publicly when it becomes available to him. ....
 No Alcohol For 2 Months After Covid Vaccine Shot, Experts Tell Why
No Alcohol For 2 Months After Covid Vaccine Shot, Experts Tell Why
Soon after Russian officials warned its citizens to avoid alcohol for two months after getting the Sputnik V vaccine shot, health experts in India said on Thursday that the move is a preventive measure for the Covid-19 patients. ....
 Online Covid Calculator Will Tell Who Must Get Vaccines First
Online Covid Calculator Will Tell Who Must Get Vaccines First
Researchers, including one of Indian-origin, have developed a new online calculator for estimating the individual and community-level risk of dying from Covid-19. ....
 Trump Rejects Early Covid-19 Vaccinations For White House Staff
Trump Rejects Early Covid-19 Vaccinations For White House Staff
US President Donald Trump has said that White House staffers should not be among the first in the country, currently the hardest-hit by the pandemic, to receive the coronavirus vaccinations. ....

